

The absorbable craniomaxillofacial fixation plate is specifically designed for facial fracture fixation, naturally degrading postoperatively to eliminate secondary removal surgery.
Item No :
GOHE008MOQ :
10 PiecesClassification :
Class IIIColor :
No ColorOrigin :
Xiamen, ChinaPayment :
T/T 50% and balance before shipmentLead Time :
Depends on the order circumstancesAbsorbable Craniomaxillofacial Fixation Plate
The absorbable craniomaxillofacial fixation plate is precision-engineered from medical-grade poly-L-lactic acid (PLLA), providing a stable and reliable internal fixation solution for maxillofacial fractures. The PLLA material exhibits outstanding biocompatibility and controllable degradation characteristics, with its degradation profile (12-24 months) perfectly synchronized with the bone healing process to ensure sustained mechanical support throughout the healing period.
This innovative product eliminates all complications associated with traditional titanium plates and screws, including foreign body sensation, thermal sensitivity, and the risks of removal surgery. It is particularly suitable for complex fractures (zygomatic/mandibular) and orthognathic surgeries. Upon completing its fixation function, the PLLA material completely degrades via natural metabolic pathways into carbon dioxide and water, leaving no trace behind.
Indications for Absorbable Craniomaxillofacial Plate:
- Fixation of maxillofacial fractures;
- Orthognathic surgery;
- Craniomaxillofacial reconstruction surgery;
- Pediatric maxillofacial trauma.
Specifications of Absorbable Craniomaxillofacial Plate:
| Product Description | Model Specifications | Illustration |
|
Straight Bone Plate |
PLLH-Z02-14 |
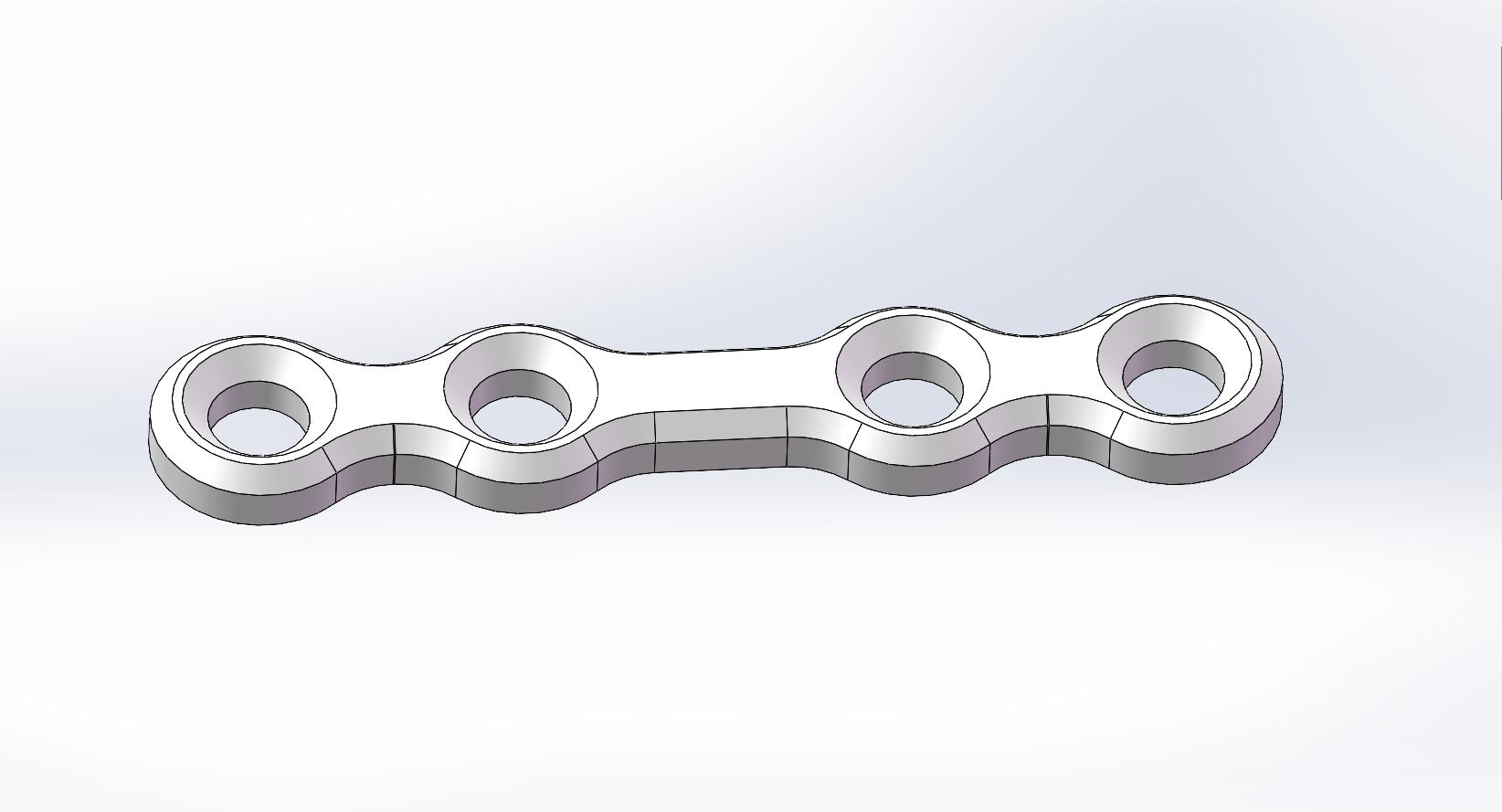 |
|
PLLH-Z02-17 |
||
|
PLLH-Z04-23 |
||
|
PLLH-Z04-25 |
||
|
PLLH-Z04-28 |
||
|
PLLH-Z04-31 |
||
|
PLLH-Z06-34 |
||
|
PLLH-Z06-37 |
||
|
PLLH-Z06-40 |
||
|
PLLH-Z06-43 |
||
|
PLLH-Z08-47 |
||
|
PLLH-Z10-59 |
||
|
Curved Bone Plate |
PLLH-H04-22 |
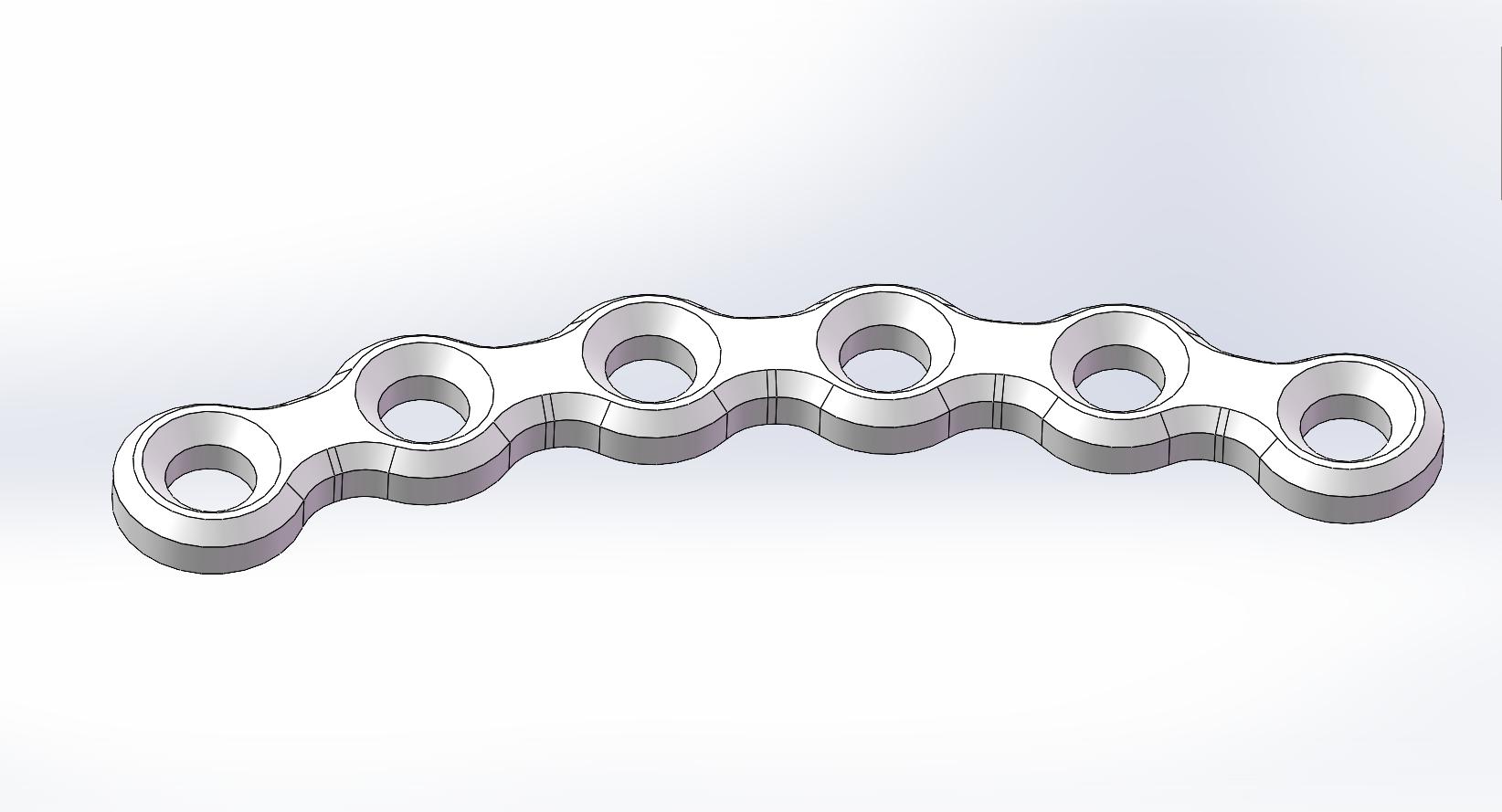 |
|
PLLH-H04-28 |
||
|
PLLH-H06-34 |
||
|
L-Shaped Bone Plate |
PLLH-L04-17L |
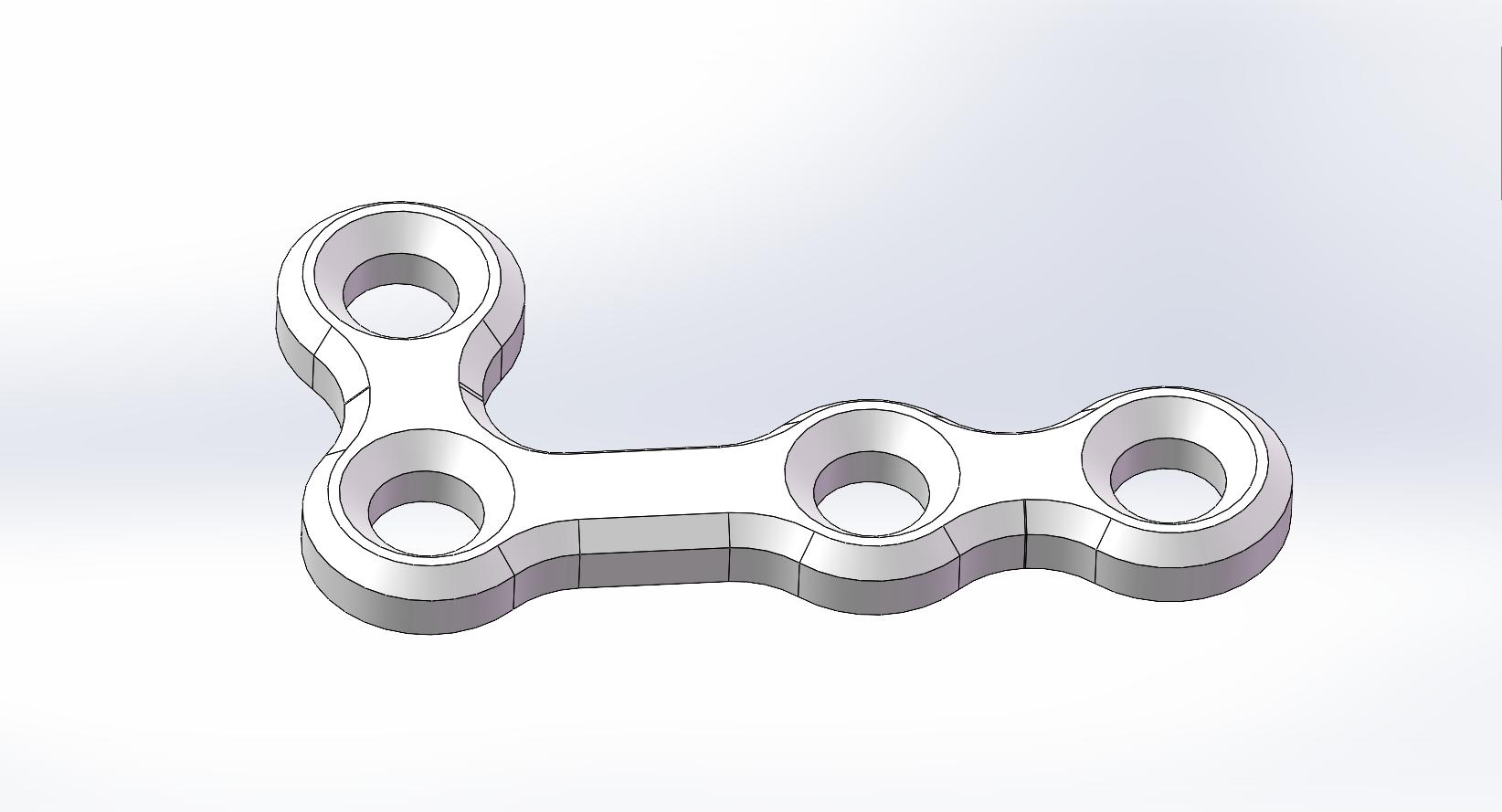 |
|
PLLH-L04-17R |
||
|
PLLH-L04-20L |
||
|
PLLH-L04-20R |
||
|
PLLH-L04-23L |
||
|
PLLH-L04-23R |
||
|
PLLH-L04-26L |
||
|
PLLH-L04-26R |
||
|
PLLH-L05-32L |
||
|
PLLH-L05-32R |
||
|
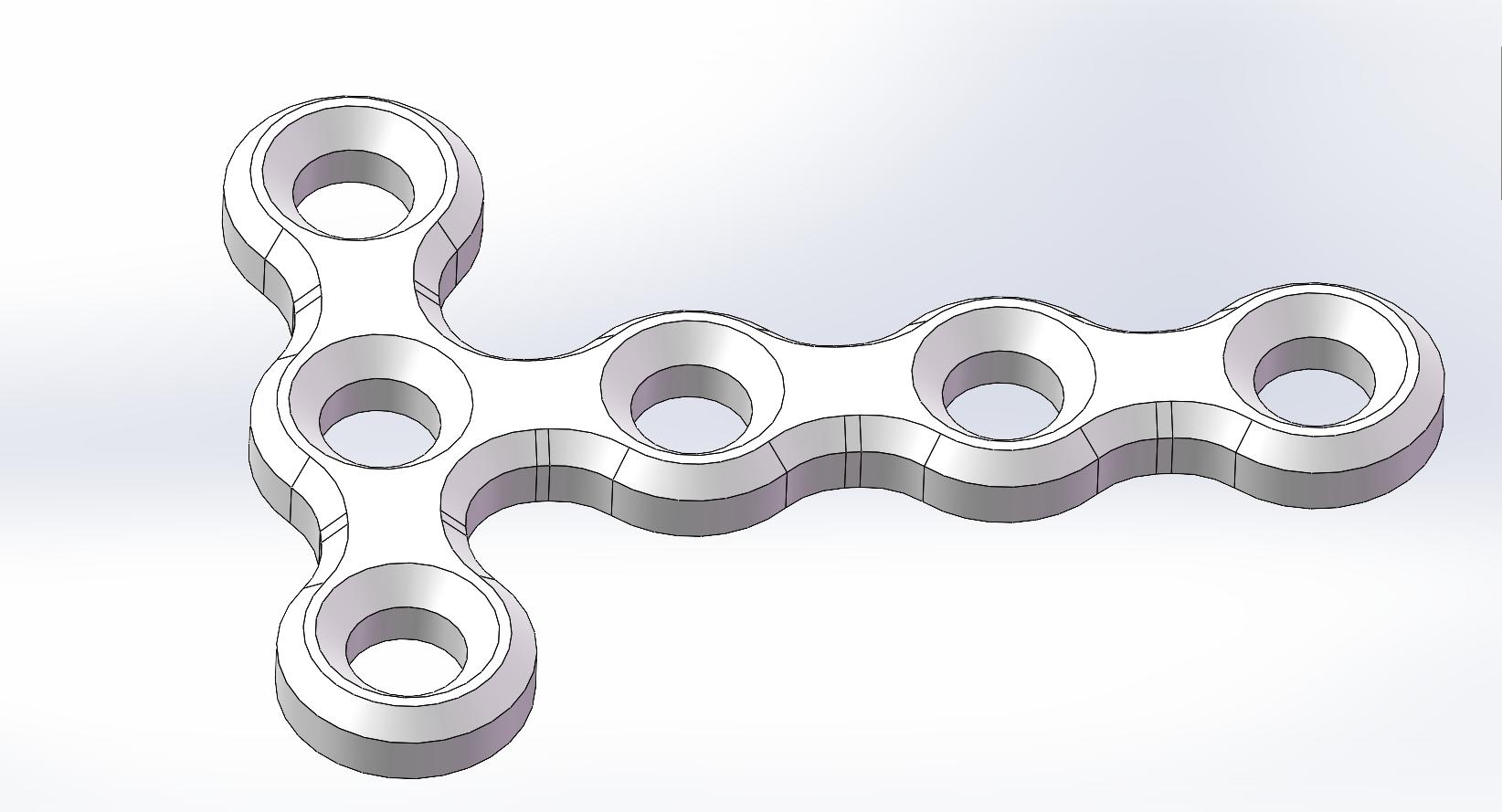
T-Shaped Bone Plate |
PLLH-T05-17 |
 |
|
PLLH-T05-20 |
||
|
PLLH-T05-23 |
||
|
PLLH-T06-23 |
||
|
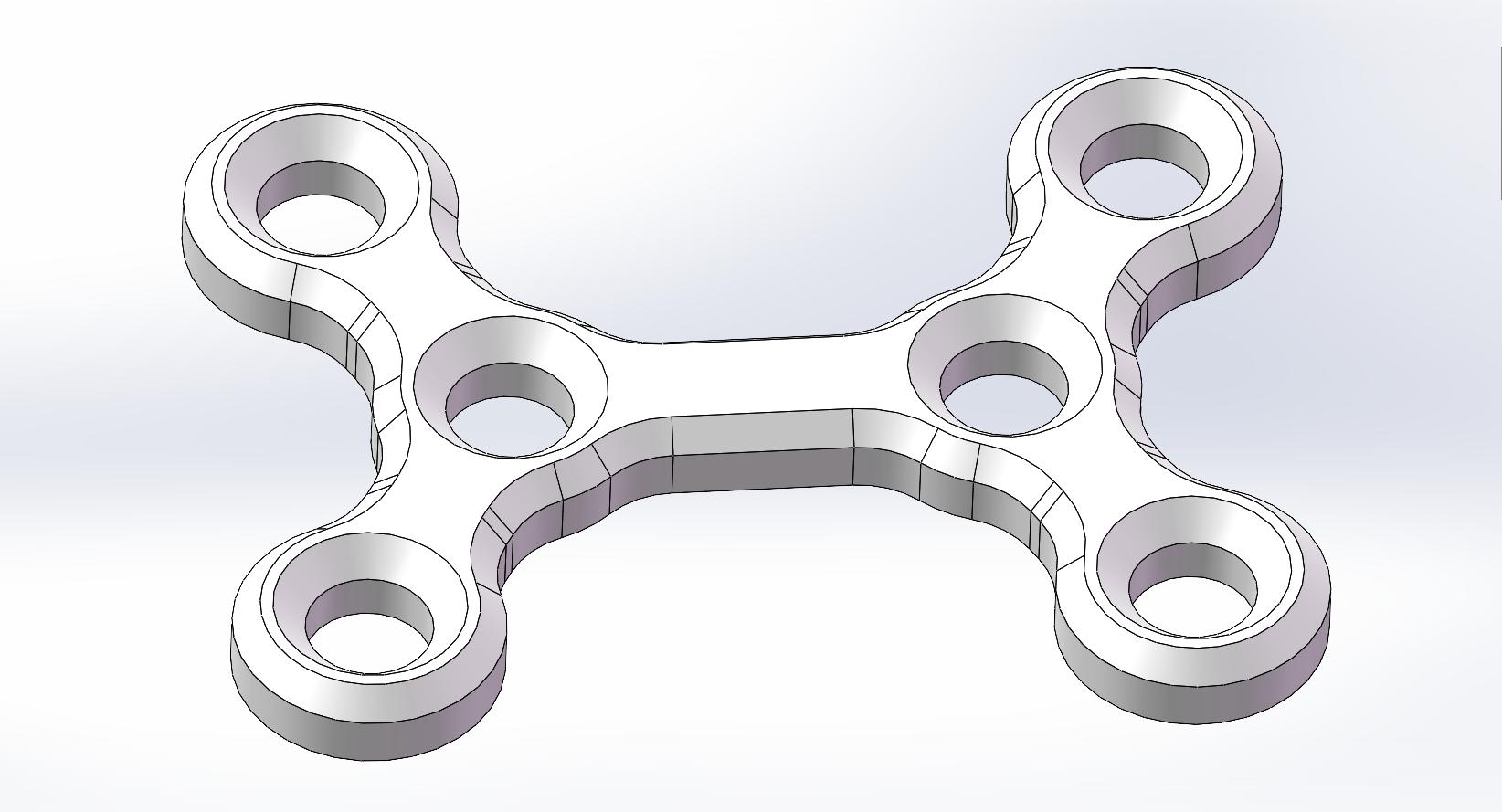
X-Shaped Bone Plate |
PLLH-X06-17 |
 |
|
PLLH-X06-20 |
||
|
PLLH-X06-23 |
||
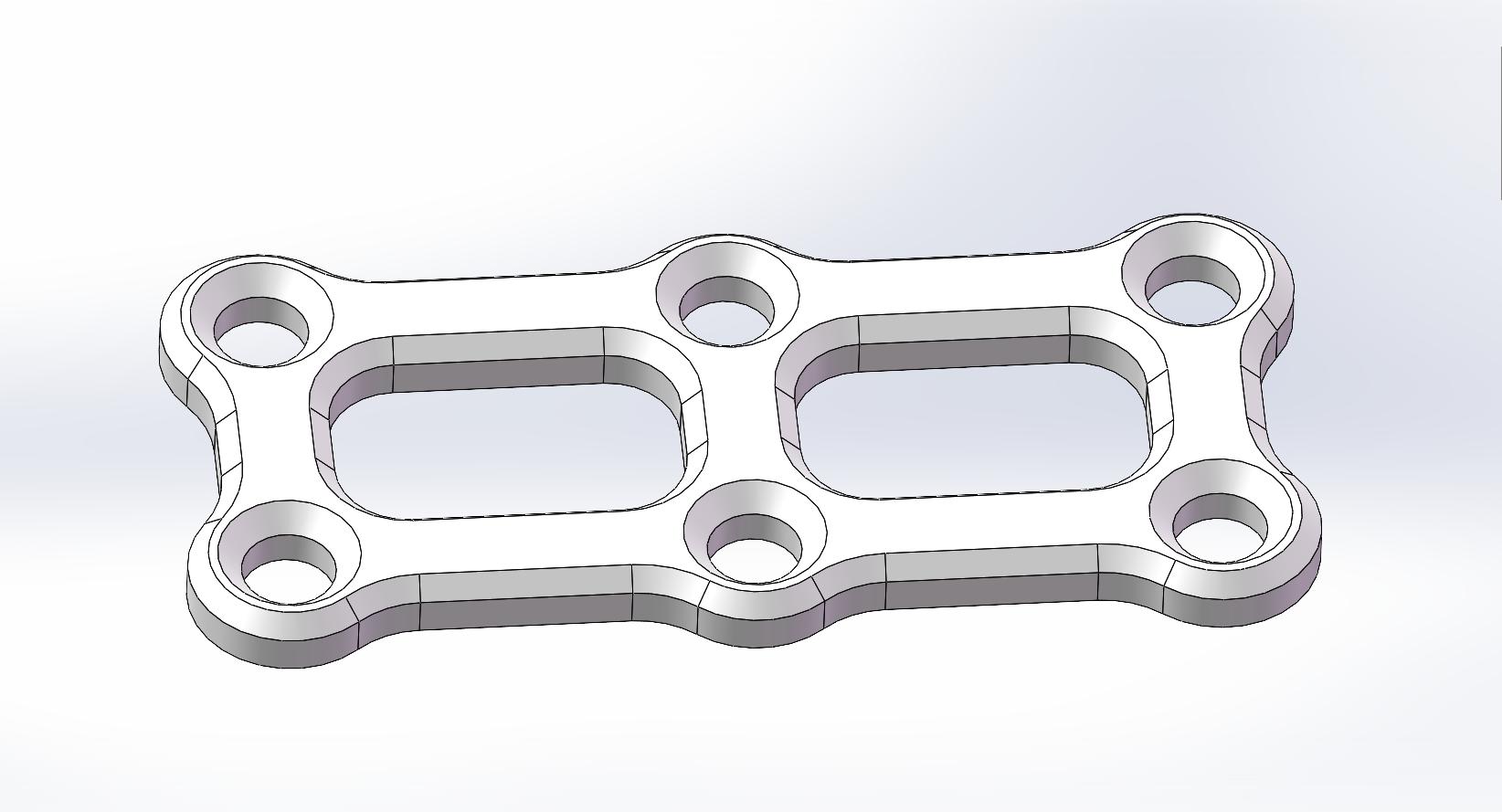
| Square Bone Plate |
PLLH-F04-16 |
 |
|
PLLH-F06-28 |
FAQ:
Q1: What is an absorbable craniomaxillofacial plate?
A1: It is an implant used for the fixation of facial and cranial bone fractures, designed to degrade naturally in the body over time without requiring removal surgery.
Q2: How does it differ from conventional metal plates?
A2: Absorbable plates eliminate the need for secondary removal procedures and do not interfere with CT or MRI imaging, making postoperative evaluation easier.
Q3: Does the plate provide sufficient mechanical strength during healing?
A3: Yes. The plate is engineered to maintain adequate stability during the critical healing period (typically 3 to 6 months), after which it gradually degrades.
Q4: When is the use of absorbable plates not recommended?
A4: In high-load areas such as the mandibular load-bearing zone, permanent fixation with titanium plates may be preferred by the surgeon.
Q5: Is there any special postoperative care required?
A5: Postoperative care is similar to that of metal plates. However, patients should avoid excessive local pressure or trauma and attend regular follow-ups to monitor absorption and healing.